ESR12 Project
Carboranylphosphines meet dendrimers: Electron-deficient scaffolds for ligand design and applications in catalysis
Diploma-delivering institutions: UPS, ULEI
Thesis co-directors: Anne-Marie Caminade (LCC, Toulouse, FR), Evamarie Hey-Hawkins (ULEI, Leipzig, DE)
Secondment hosts: BASF (DE), UAB (ES)
Academic secondment: UAB (Barcelona, ES)
Objectives
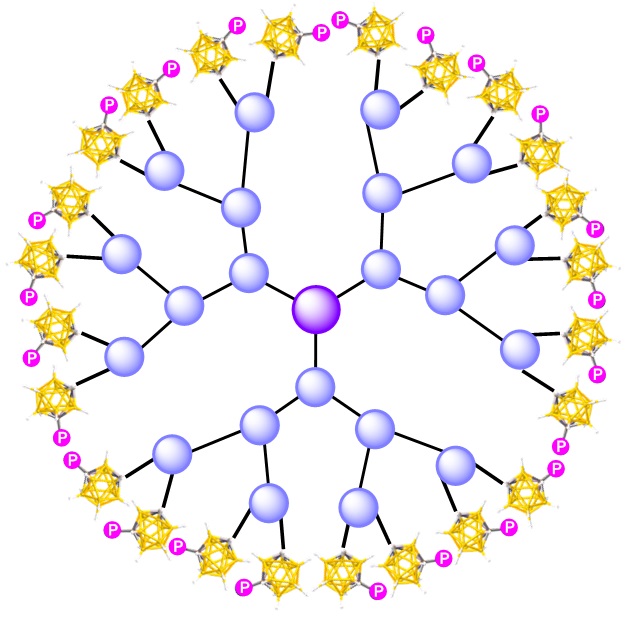
Carboranyl phosphines and phosphites, for which the Hey-Hawkins group at ULEI is the expert, will be synthesized, based in particular on 1,2 or 1,7-dicarba-closo-dodecaborane(12) [ortho– or meta-carborane(12)], which are of interest as electron-poor backbones for phosphines. Due to the tunability of the steric and electronic properties of carboranyl phosphines,[1] these ligands will be anchored to dendrimers, for which the Caminade group at LCC has vast expertise for specific applications.[2] Recently, the two groups have jointly developed redox-switchable phosphines for redox-controlled catalysis.[3]
[1] E. Hey-Hawkins et al., Boron Science, New Technologies and Applications, chapter 22, ed. N. S. Hosmane, CRC Press: Boca Raton, FL, USA, 2011, pp 513-559.
[2] A.-M. Caminade, A. Ouali, R. Laurent, C.-O. Turrin, J.-P. Majoral, Coord. Chem. Rev.2016, 308, 478-497.
[3] P. Neumann, H. Dib, A.-M. Caminade, E. Hey-Hawkins, ACIE 2015, 54, 311-314
Expected Results
We expect to obtain for the first time dendrimer-based electron-poor carboranylbisphosphines or mixed (hemilabile) carboranylphosphine-amines, ‑alkoxides, -thiols or -thioethers, etc. as ligands for challenging catalytic transformations, e.g. C–C coupling or C–H activation. The effects of different dendrimer structures and metals employed on the reactivity, activity, and selectivity of the catalysts will be studied, rationalized in collaboration with the theoretical group of Agustí Lledós (UAB) through secondment and applied for a rational design of improved catalysts. Decrease in the metal leaching will be particularly targeted, the objective being to reduce leaching below 1 ppm.

Max Milewski
PhD student - ESR12
My name is Max Milewski and I am a fellow of the CCIMC network. My research project belongs to the field of macromolecular coordination and carborane chemistry as well as homogeneous catalysis. During my time as PhD I am investigating dendrimer-based electron-poor carboranyl pnictogen and chalcogen compounds and their application in homogeneous catalysis. Different carboranylphosphines and -phosphites based on ortho- and meta-carboranes will be synthesized and combined with hyperbranched nanomolecules known as dendrimers. Anchoring both unique scaffolds the first dendrimer-based electron-poor carboranylphosphines or hemilabile carboranylphosphine-amines, -alkoxides, -thiols, -thiolethers should be synthesised and tested as ligands for challenging catalytic transformations.
Over the course of two research exchanges with Monash University in Melbourne (Australia) in the past two years, I have developed a strong appreciation for the role of transnational research in pushing the frontiers of knowledge towards more resource-efficient and sustainable synthesis. My Master’s studies in chemistry at Leipzig University included several research projects such as the synthesis and characterization of metalated propargylamines, carbone silver amides, Jacobsen-like catalysts as well as frustrated Lewis pairs incorporating nitrogen-attached 1,7-dicarba-closo-dodecaboranes(12) as precursors for homogeneous catalysis.
