ESR8 Project
Polar substrates asymmetric hydrogenation and associated processes: role of the base
Recruting institution: UoY
Diploma-delivering institutions: UoY, UPS
Thesis co-directors: Simon Duckett; Jason Lynam and John Slattery (UoY, York, UK), Rinaldo Poli (LCC, Toulouse, FR)
Secondment host: Fundacion Tecnalia Research & Innovation (ES)
Academic secondment : UAB (Barcelona, ES)
Objectives
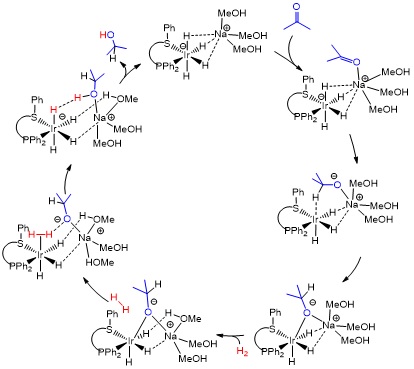
In hydrogenation and transfer hydrogenation of polar substrates, new mechanistic views have recently emerged to account for the activity of system with non-deprotonatable ligands, which nevertheless need a strong base for activity.[1] Through a combination of ligand synthesis, catalysis and mechanistic studies by advanced NMR and DFT calculations, this project targets mechanistic understanding and the development of predictive tools for optimizing the ligand structure, given specific prochiral substrates of industrial interest.
[1] (a) Dub, P. A.; Henson, N. J.; Martin, R. L.; Gordon, J. C. J. Am. Chem. Soc. 2014, 136, 3505. (b) Hayes, J. M.; Deydier, E.; Ujaque, G.; Lledós, A.; Malacea, R.; Manoury, E.; Vincendeau, S.; Poli, R. ACS Catal.2015, 5, 4368.
Expected Results
Clarification of the operating cycle for the asymmetric hydrogenation and transfer hydrogenation with Rh and Ir systems. Design of ligands able to maximize activity and enantioselectivity for target substrates. At least one catalytic system with TOF > 500 h-1 at 25°C and e.e. > 95% for a model substrate of the acetophenone family.

Paven Kisten
PhD student - ESR8
I am Paven Kisten from South Africa. I received an integrated MChem degree in Chemistry with Medicinal Science from the University of Southampton. During my time at university, my research focussed on metal-organic frameworks as heterogeneous catalysts.
First, working on nanoparticle incorporated amine functionalised frameworks for one-pot reactions under the supervision of Professor Robert Raja. Then at the University of Calgary working with Professor George Shimizu to gain an insight into a novel application of zirconium phosphonate complexes as Brønsted acid catalysts. This strengthened my ability to attune scientific procedures and sparked an interest in green chemistry.
Under the CCIMC network, my project will focus on using computational and spectroscopic techniques to revise the asymmetric hydrogenation catalytic mechanism. Through a combination of novel ligand and transition metal synthesis, we will test these catalytic systems. Enabling the creation of more robust homogeneous systems with greater yield and enantiomeric excess for the hydrogenation of prochiral substrates
